TMD Disorders can Progress to Chronic if Untreated
Acute jaw pain can advance to chronic TMD when obtaining treatment is prolonged.
Temporomandibular joint (TMJ) refers to the joint itself, while Temporomandibular disorder (TMD) refers to the various conditions and issues that may impact the functionality of the TMJ. Temporomandibular disorders (TMDs) affect close to 15% of the adult US population, [5]. To those affected by them, these medical conditions cause a significant decrease in quality-of-life because of pain and/or dysfunction and exact a high financial burden in terms of medical care.
Given this high prevalence and severity, the prevention of chronic TMDs should be a research priority, yet most of the research to date focuses on the treatment of existing TMDs. In contrast, efforts to prevent the progression from acute JAMSS injury to chronic pain are thin, consisting mostly of recommendations for jaw rest and soft diets [7]. Even though the clinical scenarios that lead to these disorders are well known, there is little awareness about what measures should be taken to mitigate the risk of chronic TMD development.
Here we present the importance of speed to treatment in the prevention of prolonged jaw dysfunction and pain. The pathogenesis of painful TMDs will be discussed, highlighting the presence of a narrow therapeutic window of time where conservative therapies have the potential to interrupt the progression of acute TMJ pain to chronic TMJ disorders.
What are Temporomandibular Disorders?
Temporomandibular disorders are a heterogeneous group of conditions that affect the TMJ and surrounding structures, such as the masticatory muscles. The Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) consists of 30 named TMD conditions, along with their diagnostic criteria.
What causes a TMD Disorder?
TMJ disorders that are muscle related are the most common cause.
The Transition to Chronicity in TMDs
To some degree, the presence of such heterogenicity limits the ability to make broad generalizations about the pathogenesis of TMDs. However, it is possible to break TMDs down into two general groups; those that affect the TMJ itself (intraarticular) and those that involve chronic pain in the masticatory tissues surrounding the joints (extraarticular).
Temporomandibular Disorders (TMD) Taxonomy
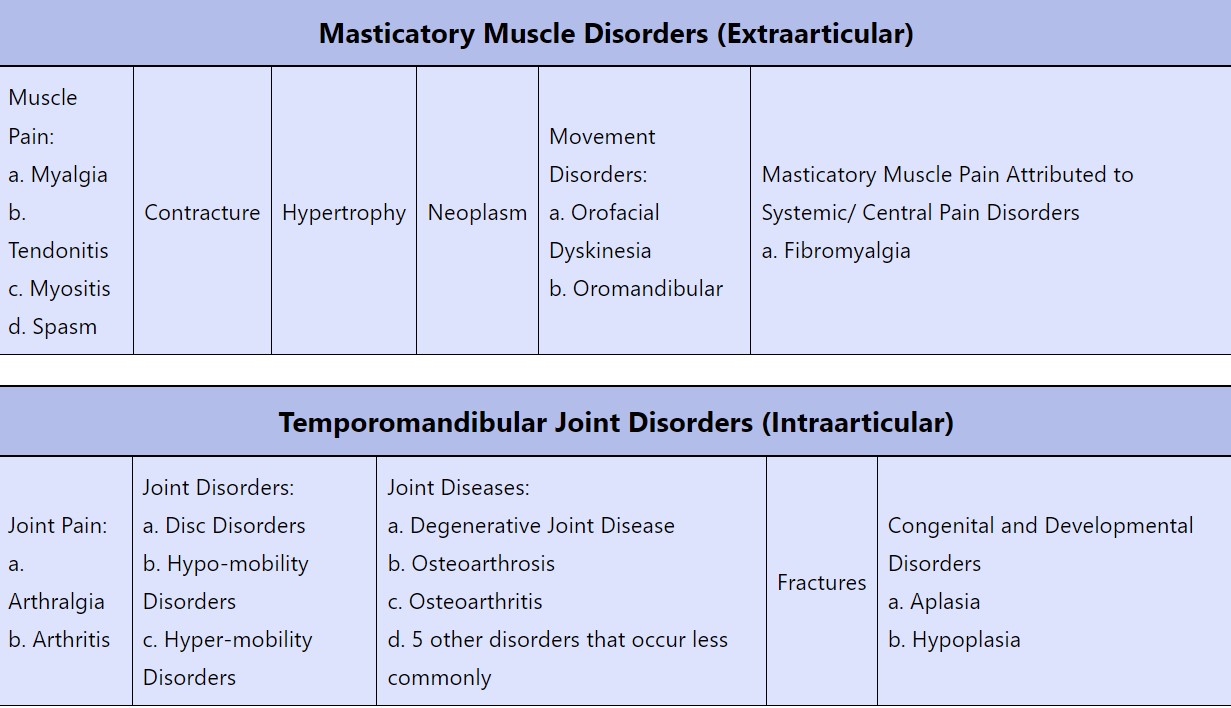
Sources: Peck et. al., 2014; Shiffman and Ohrbach, 2016 and NASEM p. 40
Myofascial TMJ Disorder Management is Best if Started Early
In the intraarticular group, structural and functional issues in the joint itself drive the disorders, with disc displacement, joint derangement, and arthritic degeneration being common manifestations. Joint-related disorders can present with or without pain and they tend to follow the disease course of other joint disorders. Many intraarticular disorders are not amenable to preventative strategies by their nature, as is the case with arthritic or degenerative processes.
In the extraarticular group, sometimes called myofascial temporomandibular disorders, the primary feature is chronic pain in the masticatory tissues, with or without related joint dysfunction. Myofascial TMDs represent the majority of TMD cases, and their pathophysiology is similar to other chronic pain disorders (such as low back pain). The pathogenesis of extraarticular TMDs is our primary focus because timely therapeutic intervention in acute injury scenarios may modify the conditions that predispose to chronic pain, making prevention strategies feasible.
How long does the acute phase of a painful TMD last?
We know that roughly 50% of people with acute jaw and muscle sprain/strain symptoms will have symptoms at the 6-month mark after injury.
Dr. G D Slade also reported that during patient follow-up, “4% of participants per annum developed clinically verified TMD, although that was a ‘symptom iceberg’ when compared with the 19% annual rate of facial pain symptoms”. The June 23, 2016 Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies article discusses that when studying the origins of a persistent condition, one must first start with the acute injury or trauma and then identify what factors are permissive to the development of chronicity.
Yet this statistic does not offer a mechanistic view of how acute conditions become chronic. As the landmark National Academies of Sciences, Engineering, and Medicine (NASEM) report on TMDs points out, “there is no general agreement regarding when the acute phase of a painful TMD ends and when chronic begins. The consequence is that the end of acute TMD pain is most often indeterminate”.
This poor understanding of the transition to chronicity in TMDs is likely responsible for the lack of cohesive preventative strategies. However, progress in elucidating the pathogenesis of painful TMDs has been made, in part because the underlying pathophysiology so closely resembles that of other chronic pain disorders. By studying what is known about the transition from acuity to chronicity, a logical and systematic approach to chronic TMD prevention can be established.
Peripheral and Central Sensitization for Temporomandibular Disorders
The model for chronic pain concerns the interplay of the peripheral and central nervous systems. The archetypal model is one that follows this sequence: first, an acute injury, next a guarding reflex in response to this injury, and finally, central sensitization of neural pain pathways secondary to the prolonged peripheral pain stimulus.
The physiologic hallmark of chronic pain is this final step, central sensitization. Once this takes place, the necessary condition is set for chronicity, and recurring or prolonged cycles of pain can be expected to occur.
Steps in the Model for Chronic Jaw Pain:
- Acute injury triggers afferent nociceptive signals in the trigeminal nerve. Release of local tissue inflammatory mediators perpetuates and amplifies the afferent response [14]. This increased excitability of sensory nerve endings at the site of injury is called “peripheral sensitization”.
- Central processing of peripheral pain signals triggers a guarding reflex in the masticatory muscles as a protective response. This modification can be seen in surface electromyographical activity in people with painful TMDs [16]. Further, the degree of muscle tension is highly correlated with subjective pain scales [5]. Constant muscle tension in the masticatory muscles causes decreased tissue oxygenation and muscle fatigue, ultimately leading to the local release of neuroactive and proinflammatory cytokines [9], creating further peripheral sensitization of painful afferent signals.
- Sustained peripheral pain signaling leads to a hyperexcitable state in the central nervous system (CNS) called “central sensitization”. The definition of central sensitization given by the NASEM (2020) is “the prolonged and increased excitability of neurons and increased synapse function in central nociceptive pathways, which can result in pain experienced that may not match or require a noxious stimulus”. Woolf [17] further describes it thus: “Central sensitization [is] where the CNS can change, distort, or amplify pain, increasing its degree, duration, and spatial extent in a manner that no longer directly reflects the specific qualities of peripheral noxious stimuli, but rather the particular functional states of circuits in the CNS… This does not mean that the pain is not real, just that it is not activated by noxious stimuli.” Central sensitization is the physiologic hallmark of chronic pain.
- Proposed mechanisms for the development of central sensitization include activation of central microglial cells [12] and neuroplastic changes to pain pathways [3]. Both of these changes are not instantaneous, but take place over a period of days to weeks.
- Psychological responses to emerging chronic pain (such as stress or anxiety) amplify central sensitization and stabilize neural pain signaling pathways. Amplification of pain due to psychological stress is mediated through the neuroendocrine system. For instance, stress triggers the hypothalamus-pituitary-adrenal (HPA) axis and the release of cortisol and pro-inflammatory cytokines, which in turn amplify and perpetuate pain signaling pathways [15].
- Any further sensory signaling from the trigeminal nerve now triggers a pain response in the CNS, even if the stimulus is normal. Clinically, this is manifested as hyperalgesia, allodynia, and referred pain [12]. In turn, constant central pain signaling perpetuates tonic guarding activity in masticatory muscles, providing further perpetuation of peripheral pain signaling. At this point, a self-reinforcing cycle of pain is established between peripheral and central sensitization and the transition from acuity to chronicity is complete.
A Jaw Injury’s Narrow Therapeutic Window
The best remedy for acute TMD is simple treatment started as soon as possible.
There is a narrow therapeutic window to prevent the establishment of prolonged pain and/or dysfunction. If a jaw injury or insult is promptly treated in the acute state, the progression to “central sensitization”, and therefore, chronic pain, may be avoided. Speed to treatment is critical to accomplish this intervention.
The best study to date that highlights the importance of speed to treatment is the landmark OPPERA (Orofacial Pain: Prospective Evaluation and Risk Assessment) study. One aspect of this study was the evaluation of patients with new onset TMJ pain that lasted for ?5 d per month for ?1 month during a 3-month reporting period. The finding was that for these patients, close to 50% had persistent TMJ pain after 6 months (by definition, a chronic TMD).
The implication is that there is a narrow therapeutic window for the prevention of chronic TMDs and it appears to be the first 1-4 weeks after injury. This corresponds well with the proposed physiological mechanisms behind central sensitization, microglial activation, and neuroplastic adaptation. Certainly, each individual case will be different, but the principle of prompt treatment to avoid central sensitization is clear.
The Multimodal Therapeutic Approach to TMD Treatment
There are three principal steps in the progression to central sensitization that are good targets for therapeutic intervention:
- Peripheral sensitization: Decreasing peripheral pain signaling can be conservatively addressed via thermotherapy, cryotherapy, and oral analgesics. Rest strategies (such as a soft food diet, and avoiding gum chewing) can also decrease peripheral pain signaling.
- Muscle guarding: Guarding is a key step in the perpetuation of the cycle of pain. Methods to address muscle guarding include oral appliance use, cryotherapy, thermotherapy, and physical therapy exercises.
- Psychological stress: Anxiety and psychological stress are accelerants to central sensitization. Pain management therapy helps relieve these factors, making it an excellent adjuvant preventative strategy.
Non-invasive different modalities of treatment for temporomandibular disorders
Non-surgical options for TMD Disorders can halt the cycle of increasing chronic jaw pain.
It is our contention that a multimodal approach, using non-invasive and complementary measures, provides the best chance of stopping the cycle of pain amplification and progression to chronicity. Based on the above targets, we advocate for the use of the following conservative interventions.
Types of conservative TMD Disorder Interventions:
1. Oral Appliances
The use of oral appliances for chronic TMD treatment is widely accepted. However, most designs are geared towards treating existing disorders rather than preventing new ones. If the main goal of an oral appliance is to mitigate the deleterious effects of the guarding reflex, the key function of the appliance should be joint unloading.
The imperative of speed to treatment is accomplished by our Hegab Splint (TMJ splint therapy , Hegab TMJ Splint (HTS) , MRI-based determination of occlusal splint thickness for temporomandibular joint disk derangement: a randomized controlled clinical trial ) . The splint is fabricated at our center by the professional doctor and can be used immediately. Hegab Splint can immediately protect the muscles and joints and improve all the patients symptoms
2. Cryotherapy
Cryotherapy (cold therapy) is a well-established treatment for acute orthopedic injuries. Cold decreases circulation to the injured region, slowing the inflammatory reaction and reducing inflammation. Cold also has an analgesic effect by slowing nociceptive conductivity and decreasing neuronal excitability. Cold also has the effect of decreasing muscle tension [10].
3. Thermotherapy
Thermotherapy (heat therapy) is also well-established and is typically used several days after the initial injury. Thermotherapy increases circulation and oxygenation and allows for the elimination of metabolic waste. It also reduces nerve pain conduction. Finally, heat promotes muscle relaxation, facilitating ease of mobility and the reduction of the guarding reflex [4].
4. Physical Therapy:
Physical therapy exercises are considered front-line therapy for chronic TMDs. In the acute setting, early mobility is important for the interruption of peripheral sensitization. Multiple studies have shown that jaw exercises facilitate pain-free mouth opening and reductions in disturbances to daily living [2].
5. Pain management:
There are multiple different approaches to pain management, such as cognitive-behavioral therapy (CBT) and biofeedback. Multiple studies have shown that therapist-directed pain management is effective in reducing multiple measures of pain [1]. These findings provide support for the use of therapist-directed pain management in the acute setting to prevent the onset of chronic pain.
CONCLUSION: TMD Disorders Classification, Development, and Early Treatment
The pathogenesis of chronic pain indicates that there are several potential targets that may interrupt the transition from acute to chronic pain. The key principle in preventing chronicity is speed to treatment. The primary preventive strategies include reducing peripheral sensitization, preventing chronic jaw muscle tension and the guarding reflex, and relieving the psychological states that accelerate central sensitization.
The presence of multiple targets calls for a multi-modal therapeutic strategy. This multi-modal approach must be patient-directed, easily followed, and immediately deployable without specialist intervention.
References
[1] Aggarwal VR, Fu Y, Main CJ, Wu J. The effectiveness of self-management interventions in adults with chronic orofacial pain: A systematic review, meta-analysis, and meta-regression. European Journal of Pain. 2019;23(5):849–865.
[2] Armijo-Olivo S, Potance L, Singh V, Neto F, Thie N, Michelotti A. Effectiveness of manual therapy and therapeutic exercise for temporomandibular disorders: Systematic review and meta-analysis. Physical Therapy. 2016;96(1):9–25.
[3] Chichorro JG, Porreca F, Sessle B. Mechanisms of craniofacial pain. Cephalalgia. 2017;37(7):613–626.
[3] Chichorro JG, Porreca F, Sessle B. Mechanisms of craniofacial pain. Cephalalgia. 2017;37(7):613–626. [4] Furlan, et al. The use of superficial heat for treatment of temporomandibular disorders: an integrative review. Codas 2015;27(2):207-12.
[5] Gauer, et al. Diagnosis and Treatment of Temporomandibular Disorders. Am Fam Phys 2015; 91(6):378-386.
[6] Glaros, et al. The role of parafunctions, emotions, and stress in predicting facial pain. J Am Dent Assoc. 2005;136(4):451-8.
[7] National Academies of Sciences, Engineering, and Medicine. Temporomandibular Disorders: Priorities for Research and Care. Nat Acad Press 2020; 3.
[8] Human Pain Genetics Database: A resource dedicated to human pain genetics research. Pain. 2018;159(4):749–763
[9] Jounger SL, et al. Increasd levels of intramuscular cytokines in patients with jaw muscle pain. J Headache Pain. 2017;18(1):30.
[10] Kopacz L, et al. Comparative Analysis of the Influence of Selected Physical Factors on the Level of Pain in the Course of Temporomandibular Joint Disorders. Pain Res Manag. 2020; 2020:1036306.
[11] Levine JE. Chapter 1, an introduction to neuroendocrine systems. In: Fink G, Pfaff DW, Levine JE, editors. Handbook of neuroendocrinology. San Diego, CA: Academic Press; 2012. pp. 3–19.
[12] Ma F, Zhang L, Lyons D, Westlund KN. Orofacial neuropathic pain mouse model induced by trigeminal inflammatory compression (TIC) of the infraorbital nerve. Molecular Brain. 2012;5:44.
[13] Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318.
[14] Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. International Review of Neurobiology. 2011;97:179–206.
[15] Staniszewski K, Lygre H, Bifulco E, Kvinnsland S, Willassen L, Helgeland E, Berge T, Rosén A. Temporomandibular disorders related to stress and HPA-axis regulation. Pain Research & Management. 2018;2018:1–7.
[16] Szyszka-Sommerfeld, et al. The Diagnostic Value of Electromyography in Identifying Patients With Pain-Related Temporomandibular Disorders. Front Neurol. 2019; 10:180.
[17] Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15.