Headache TMD Studies Show Relationship

Headache’s Connection to Temporomandibular Joint Disorders
The connection between headaches and temporomandibular disorders (TMDs) is well established.
Recent advances in the neurobiology of headaches and TMDs help explain a strong epidemiological association and bidirectional causality. Headache TMD studies explore the joint pathophysiology of headache and TMDs, discuss the clinical management of jaw-injury issues, and describe a conservative approach to TMD care that can complement most headache treatment regimens.
Statistical Evidence of Headache Symptoms and their Relationship to TMJ
NIH study of headaches and their contribution to 1st-onset TMD
Among patients seeking medical care for headaches, the prevalence with patients with TMD symptoms ranges from 52-55% in population studies. According to Richard K. Ohrbach, DDS, PhD, et al in the January, 2017 Temporal change in headache and its contribution to risk of developing first-onset TMD in the OPPERA study article, “Patients with TMD were more likely to experience worsening in the headache type compared with that by controls, eg, prevalence of definite migraine among TMD cases increased 10-fold”.
When segregated into painful vs. functional TMDs, the prevalence in the pain-related TMD group increases substantially, greater than 80% in a recent NIH observation study. [1] Several headache subtypes have been diagnosed: self-reported headache, (probable) migraine, (probable) tension-type headache, and secondary headache recognized for its connection to TMD. TMD occurrences were subdivided into 2 subtypes: painful TMD and function-related TMD.
2021 Journal of Headache and Pain report
Headache severity is also linked to the number of TMD symptoms. In a 2010 analysis, the prevalence of headache in TMD patients with one symptom was 57%, with two symptoms was 65%, and with three or more symptoms was 73%. A 2021 Journal of Headache and Pain report says that TMD symptoms were greater with an increased frequency of temple headache, and the presence of TMD was associated with a greater frequency of migraine episodes. A US population study demonstrated a five-fold higher prevalence of TMD symptoms in people with severe headache compared to those without severe headache. [2]
Temporomandibular Disorders & Headache: A 2017 NIH Retrospective Analysis
The 2014 Diagnostic Criteria for TMD (DC/TMD) introduced a novel diagnostic category, Headache Attributed to TMD (HATMD), a change that acknowledges that TMDs can be an important cause of headaches. This novel category describes headaches in the temple area that can be replicated by the provocation of the TMJ system. The International Classification of Headache Disorders, 3rd edition (IDCH-3) adopted this new diagnosis with the caveat that TMD symptoms should be antecedent to the headaches.[3]
However, the literature also suggests that this causal relationship can be reversed. The Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study showed that headaches are a significant risk factor for the development of first-onset TMD symptoms. Further, when comparing those who developed TMDs versus controls, headache severity, progression, and frequency were elevated in the TMD group. Of note, the prevalence of definite migraine episodes increased 10-fold in the TMD group versus controls.
How are the Pathophysiology of Headache and TMD Related?
To outline how headache and TMD pathogenesis are related, it’s helpful to start with migraine headaches. The trigeminovascular system is the rich innervation of meningeal, dural, and pial tissues by trigeminal perivascular neurons. The classic vascular thesis of migraine is that vasodilation of intracranial vessels is responsible for pain generation. However, recent research suggests that vasodilation is an epiphenomenon and that peripheral and central sensitization in the trigeminal nociceptive system is the more likely cause of migrainous pain. [4]
This paradigm shift was accompanied by an increased understanding of the role of calcitonin gene-related peptide (CGRP) in migraine pathogenesis. This neuropeptide is abundantly distributed in the central and peripheral nervous system and pain pathways. It is believed to cause neurogenic inflammation and is co-localized with several other biomarkers of pain. [5]
It appears that most migraine drugs have a mechanism of action that decreases CGRP activation, either indirectly or directly
The impact of migraine drugs on lowering CGRP activation:
- Ergotamines and triptan migraine drugs stimulate 5-HT receptors, which has an indirect effect of reducing the release of CGRP, resulting in reduced trigeminal activation and vasodilation.
- Direct blockade of CGRP signaling with monoclonal antibodies and other compounds is effective in treating acute migraine. [6]
- Botulinum toxin (Botox) suppresses CGRP levels in the trigeminovascular system, suggesting that CGRP reduction is its mechanism of action.
Additional findings of the relationship between migraines and CGRP include:
- Levels of CGRP in saliva, cerebrospinal fluid, and peripheral blood are elevated during severe migraine attacks. [7]
- Clinical improvement of migraine symptoms after triptan administration is accompanied by decreased CGRP levels. [8]
- Subjects injected with CGRP triggers migraine headaches in both healthy subjects and migraineurs.
- Subjects with chronic migraine have higher levels of CGRP in peripheral blood samples than those with episodic migraines. [9]
- CGRP antagonists can abort migraines. [8]
Aside from its role in acute migrainous headaches via the trigeminovascular system, CGRP also is implicated in the transition to chronic headaches through its promotion of peripheral and central sensitization. Peripheral and central sensitization are the primary mechanisms behind chronic pain syndromes, including headaches and TMDs.
The process of how headaches and TMD connect in the human body:
1. Peripheral injury triggers pain signals in the trigeminal nerve. Local tissue inflammation releases cytokines and pro-inflammatory mediators that perpetuate and increase the pain response. Peripheral sensitization lowers the depolarization threshold, causing normal stimulation to be perceived as painful, a condition called “hyperalgesic priming”.
2. Sustained peripheral pain signaling leads to central sensitization, resulting in increased excitability of central pain pathways. At first, this sensitization is activity-dependent and consists primarily of lowered depolarization thresholds. If this persists for days to weeks, it evolves into an activity-independent phenomenon through neuroplastic adaptation in neuronal, glial, and immune cells. Central sensitization is the physiological hallmark of chronic pain syndromes and is responsible for the clinical symptoms of hyperalgesia and allodynia.
CGRP as a factor in Pain Hypersensitivity
Understanding the overall role of CGRP in peripheral and central sensitization appears to be through its neuroinflammatory and vasodilation effects. It causes the release of nitrous oxide and sensitizes neurons through stimulation of inflammatory mediators in the periphery, the trigeminal ganglion, in secondary connections in the trigeminal nucleus caudalis, and tertiary connections in the thalamus, limbic system, and sensory cortex. [8] Experimental evidence shows that intrathecal administration of CGRP levels stimulates central supporting cells (microglial cells and astrocytes) to release pro-inflammatory mediators that are known to induce and perpetuate central sensitization.
Current research suggests that CGRP is the molecular link between migraine headaches and TMDs. The connection can be illustrated by a popular animal model for migraines. In this model, the masseter muscle of animals is injected with a chemical irritant to trigger a peripheral inflammatory response. This inflammatory response causes a rapid and significant increase in CGRP mRNA in the trigeminal ganglion19. The result of this increased CGRP expression is a dural-trigeminovascular response that is migraine-like, a feature that is used to model migraines in preclinical experiments. [10]
In summary, CGRP is implicated as the primary activating factor of migraine headaches via stimulation of peripheral and central sensitization, both in the acute activity-dependent phase and the chronic activity-independent phase. While much less is known about tension-type headaches and other chronic headaches, central sensitization of the trigeminal system is considered to play an important role. [8]
Can Inflamed Masticatory Muscles Trigger Migraine Headaches?
Yes. Researchers now believe that this can be explained by cross-excitation in the trigeminal ganglion. That peripheral trigeminal inflammation in masticatory muscles can trigger migraine pathology is a fascinating phenomenon.
To understand how this happens, it helps to first review trigeminal anatomy. The peripheral afferents of both meningeal tissues and the TMJ apparatus project towards the trigeminal ganglion, the location of their cell bodies. Neural connections from the ganglion then project to the trigeminal nucleus caudalis, which in turn has tertiary connections in the thalamus, cortex, and limbic system. [11] Hence, the area of interest where both neural systems project is the trigeminal ganglion.
As explained above, peripheral insult in the masseter causes CGRP expression in the trigeminal ganglion. When CGRP is expressed in the ganglion, it acts in a paracrine fashion by stimulating satellite microglial cells to release pro-inflammatory mediators, leading to an inflammatory cascade. One study showed that administering CGRP to migroglial cells in culture stimulates the release of over 20 cytokines. [12]
These findings explain how peripheral inflammation of trigeminal afferents from the face can stimulate peripheral sensitization of meningeal afferents, namely, through cross excitation in the trigeminal ganglion. [7] Activation in the ganglion then causes activation of central trigeminal neural projections. [9] This is also true in the opposite direction, as clinical evidence shows that migraines are a significant predictor of TMD onset.
Important research highlights from the growing body of literature on the role of CGRP in TMD pathology:
- There is a significantly higher level of CGRP in deranged TMJ joints vs. controls in human subjects, and the extent of these levels are positively correlated with pain scores.
- Injection of the TMJ capsule with CGRP stimulates the expression of proteins associated with peripheral and central sensitization in neuronal and glial cells in animal models.
- The presence of pre-existing myogenic TMD lesions causes increased central CGRP release and enhanced migraine-hypersensitivity in animal models. [8]
- Both acute and chronic arthritis are associated with significant increases in CRGP expression in the trigeminal ganglion in animal models.
When these findings are combined with those of the role of CRGP in headaches, a powerful framework for understanding the pathogenesis of headache/TMD co-morbidity emerges.
- CGRP receptors are widely distributed through the trigeminal system, including supporting cells.
- The inflammatory response from peripheral injury stimulates the expression of CGRP in the trigeminal ganglion.
- CRGP release in the ganglion stimulates the release of pro-inflammatory mediators via supporting cells.
- This causes intraganglion activation which leads to cross-excitation of peripheral and meningeal afferents.
- Ganglion excitation is perpetuated by CRGP-mediated peripheral and central sensitization in second and third-order neurons..
- Central sensitization leads to chronic pain syndromes and increased severity of the headache phenotypes.
Clinical Considerations Determining a Headache to be Secondary to TMD
The utility of both the RC/TMD designation of HATMD and its counterpart in the ICHD-3 version of the same is that it shows a headache to be secondary to a TMD, as opposed to having co-morbid primary headache and TMD.
What diagnostic criterion determines if a headache is secondary to TMD?
The criteria commonly used to diagnose the headache and TMD relationship are:
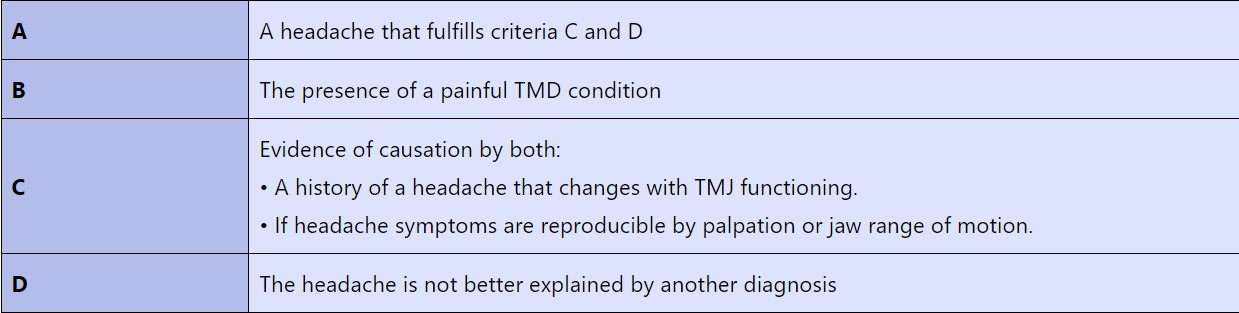
The clinical value of determining headaches as primary or secondary.
These criteria were shown to have a sensitivity of 89% and specificity of 87%. The prevalence of HATMD may be higher than previously thought. In a study of 185 patients diagnosed with both a TMD and a primary headache, it was found that 61% fit the RC/TMD diagnostic criteria for HATMD. Interestingly, HATMD was more prevalent in those with migraines (70%) than with tension-type headaches (54%). [15]
Knowing that a headache is secondary may relieve the clinician of the need to treat the headache. Clinical evidence of this is seen in a recent study of 34 HATMD patients treated only for TMD symptoms. The finding was a significant correlation between a reduction in TMD symptoms and the frequency and intensity of headaches. [15]
While there may be clinical utility in designating a headache as secondary instead of primary, the pathophysiology of headaches and TMDs describes a bidirectional and mutually reinforcing dynamic between the two conditions, regardless of the original cause.
Treatment Considerations for Patients Suffering from Headaches and TMD
The clinical goal of treating co-morbid headache and TMD is to decrease the peripheral and central sensitization that drives the pain cycle. The approach to treatment differs substantially between the two conditions due to anatomical constraints. By definition, a primary headache is an intracranial problem not due to an underlying disease or condition.
Treatment, therefore, relies on pharmacologic therapy that can access intracranial targets. While specific headache medications are outside of the scope of this review, it’s important to note that the mechanism of all appears to be related to inhibition of CGRP activation, and by extension, peripheral and central sensitization. [8]
Treatments of TMDs also target peripheral and central sensitization, but they can employ non-pharmacologic interventions because of anatomic accessibility. A conservative multimodal treatment approach for TMDs has the best chance at interrupting the pain cycle.
Suggested Treatments for TMJ and Headache Disorder include:
1- Hegab Splint Therapy
Hegab splints prevent posterior occlusal contact. This inhibits bite activation and promotes joint unloading, which facilitates masticatory muscle relaxation and pain reduction.
(TMJ splint therapy , Hegab TMJ Splint (HTS) , MRI-based determination of occlusal splint thickness for temporomandibular joint disk derangement: a randomized controlled clinical trial )
2- Cryotherapy/Thermotherapy
Cryotherapy decreases blood flow to the painful region, reduces inflammation, and decreases muscle tension. Thermotherapy increases blood flow, reduces nerve conduction, and promotes muscle
3- Physical Therapy:
Physical therapy exercises are considered front-line therapy for chronic TMDs. Exercises can be modulated to target both peripheral and central sensitization drivers of pain. [16]
4- Behavioral Pain Management:
There are multiple different approaches to pain management, many of which show significant results. While still unclear, it is thought that these therapies decrease central sensitization via their impact on the stress system and limbic activation.
In this recent study, the coexisting diagnosis of headache and TMD is difficult to separate. Headache appeared the most common symptom/disease accompanying TMD. Another important finding was the high prevalence of headache within the TMD population. This study further confirms central sensitization is present in both headache and TMDs and the study’s conclusion confirms the close relationship between these two disorders and the importance of early intervention and multidisciplinary treatment.
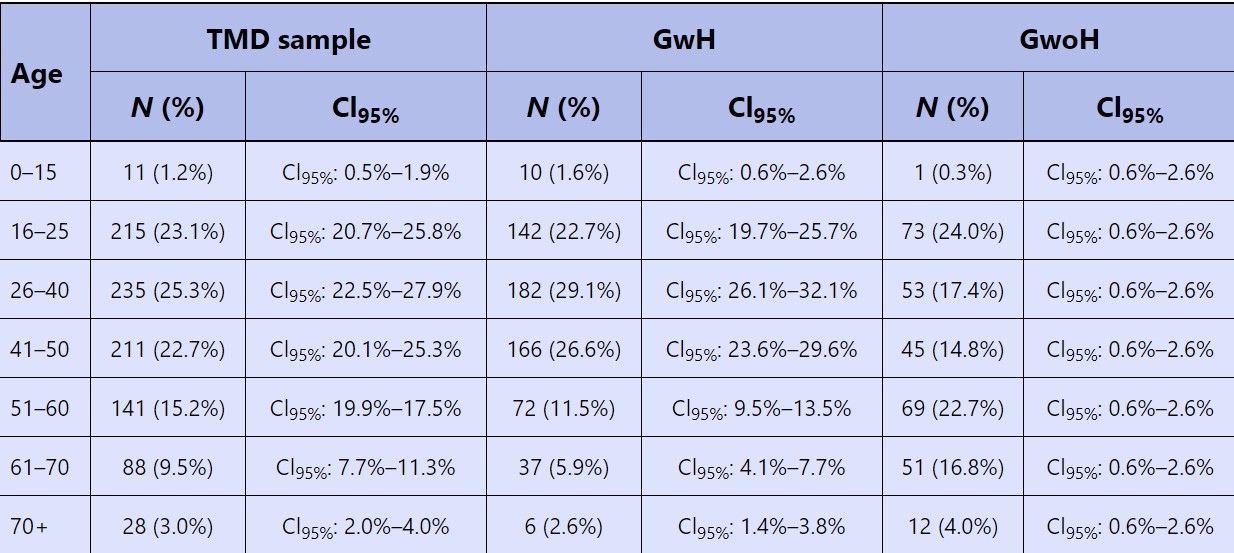
Absolute frequency (N) and percentage values of age intervals in Total Dysfunctional Sample (TDS) and in Group with Headache (GwH) and Group without Headache (GwoH).
Concluding how Headache and TMD Pathology are Linked
Headache and TMD Pathology through our human trigeminal system have a proven connection. More will become clearer with future headache TMD studies. The high degree of bidirectional causality and mutual reinforcement is due to cross excitation in the trigeminal ganglion. This excitation drives peripheral and central sensitization, the neurobiological dynamic that both pain syndromes share.
While there is some utility in determining if a headache is primary or secondary to a TMD, the pathophysiology suggests that simultaneous treatment of both conditions will yield superior results. Given that multimodal treatment of TMDs is conservative, non-pharmacologic, and patient-directed, it is reasonable to employ this treatment with all patients who have a comorbid headache and TMD symptoms, regardless of whether the headache is primary or not.
References
[1] Van der Meer HA, et al, The Association Between Headaches and Temporomandibular Disorders is Confounded by Bruxism and Somatic Symptoms. Clin J Pain. https://pubmed.ncbi.nlm.nih.gov/28002094/, January 2017
[2] Inna E. Tchivileva, et al, Clinical, psychological, and sensory characteristics associated with headache attributed to temporomandibular disorder in people with chronic myogenous temporomandibular disorder and primary headaches, https://thejournalofheadacheandpain.biomedcentral.com/articles/10.1186/s10194-021-01255-1, May 2021
[3] Schiffman E, Ohrbach R, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. doi:10.11607/jop.1151, 2014
[4] Amin FM, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. doi:10.1016/S1474-4422(13)70067-X, 2013
[5] Schou WS, et al, Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain. doi:10.1186/s10194-017-0741-2, 2017
[6] De Vries T, et al, Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. doi:10.1016/j.pharmthera.2020.107528, 2020
[7] Durham PL. Diverse Physiological Roles of Calcitonin Gene-Related Peptide in Migraine Pathology: Modulation of Neuronal-Glial-Immune Cells to Promote Peripheral and Central Sensitization. Curr Pain Headache Rep. doi:10.1007/s11916-016-0578-4, 2016
[8] Edvinsson JCA, et al. The fifth cranial nerve in headaches. J Headache Pain. doi:10.1186/s10194-020-01134-1, 2020
[9] Cernuda-Moroll?n E, et al, Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. doi:10.1212/WNL.0b013e3182a6cb72, 2013
[10] Akerman S, Romero-Reyes M. Preclinical studies investigating the neural mechanisms involved in the co-morbidity of migraine and temporomandibular disorders: the role of CGRP. Br J Pharmacol. 2020 doi:10.1111/bph.15263, 2020
[11] K?rtési T, et al, The effect of orofacial complete Freund’s adjuvant treatment on the expression of migraine-related molecules. doi:10.1186/s10194-019-0999-7, 2019
[12] Afroz S, et al. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception, doi:10.3390/ijms20030711, 2019
[13]. Shu H, et al. A Pre-Existing Myogenic Temporomandibular Disorder Increases Trigeminal Calcitonin Gene-Related Peptide and Enhances Nitroglycerin-Induced Hypersensitivity in Mice. doi:10.3390/ijms21114049, 2020
[14] Akerman S, Romero-Reyes M. Preclinical studies investigating the neural mechanisms involved in the co-morbidity of migraine and temporomandibular disorders: the role of CGRP. Br J Pharmacol. 2020 doi:10.1111/bph.15263, 2020
[15] Hara K, et al. Headache attributed to temporomandibular disorders and masticatory myofascial pain. doi:10.2334/josnusd.15-0491, 2016
[16] Fern?ndez-de-las-Pe?as C, Von Piekartz H. Clinical Reasoning for the Examination and Physical Therapy Treatment of Temporomandibular Disorders (TMD): A Narrative Literature Review. doi:10.3390/jcm9113686, 2020



